Ahead of her presentation ‘React or non-react? Insights into silica/silane/functionalized SBR compounds’ at the Tire Technology Expo Conference 2024 in Hannover, Germany (March 19, 20 & 21), Prof. Anke Blume from the University of Twente reveals the results of a study into different possible interaction/coupling reactions in a silica/silane compound with functionalized SBRs.
What will your presentation be about?
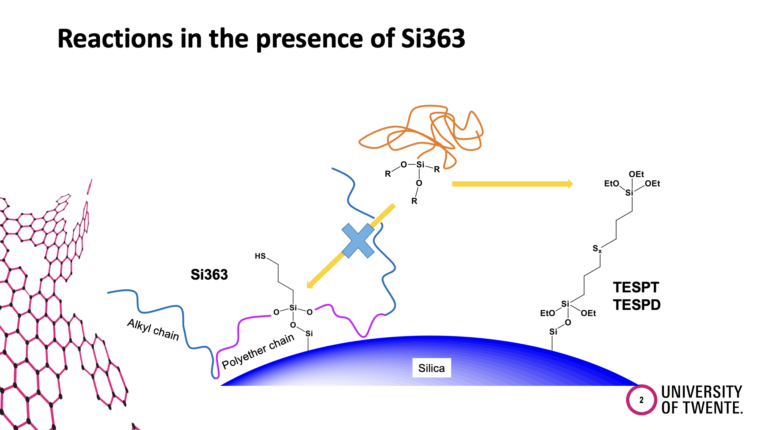
Functionalized SBR is widely used for modern passenger car tire tread compounds. The functional groups are introduced in a silica/silane-filled SSBR to enhance the polymer-filler interactions. However, what kind of reactions take place in the compound in the presence of the functionalized SBR were not clarified yet. In this study, different possible interaction/coupling reactions in a silica/silane compound with functionalized SBRs were investigated.
What examples of different possible interaction/coupling reactions will be explored?
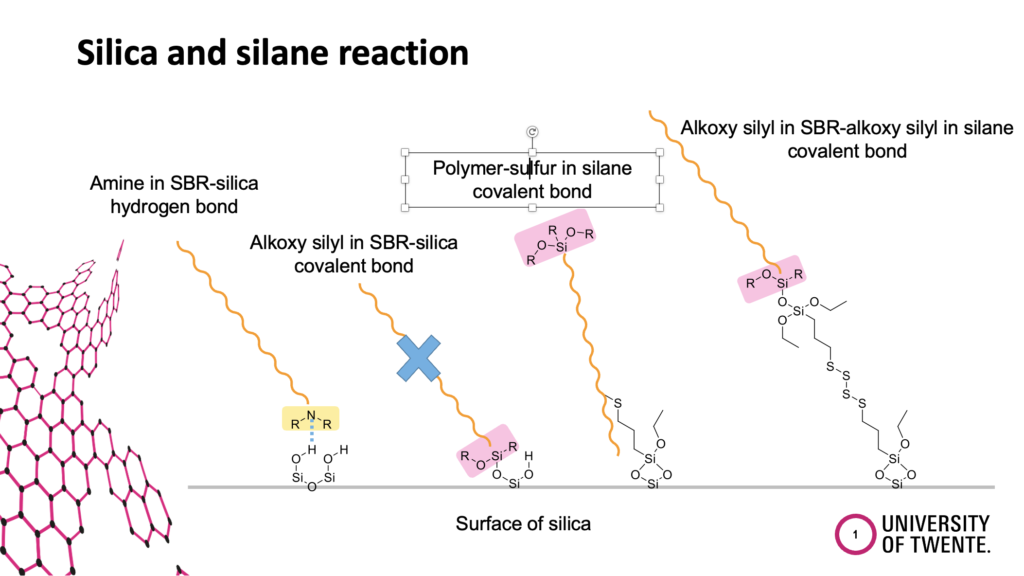
All possible physical and chemical interactions will be discussed. For example, the direct coupling of the function group at the polymer to the silica, the coupling of the functional group at the polymer via a silane to the silica, and the coupling of the silane to the functional group at the polymer.
Why haven’t these reactions been clarified before?
The introduction of functional groups at the polymer was driven by the goal to increase the polarity and therefore the compatibility of the non-polar polymer with the polar silica. The common assumption was that the functional group at the polymer couples to the silica, following the experience gained from the coupling between triethoxysilyl groups at the silane and the silanol groups at the silica. That such a functional group at the end of a polymer chain is shielded in the polymer coil and can find much easier a silane molecule beside the stiff silica particle was not considered. Anyhow, the separation of the different reactions in a clear way is also quite challenging and requires many experiments. Such a work can normally be carried out only in the frame of a PhD thesis. We were lucky to get the financial support to carry out such a study.
Why have you chosen to speak on this topic at Tire Technology Expo and why is it important at this time?
We were quite surprised about the outcome of the study and would like to share this knowledge with others. It means a change in the understanding of how functionalized SBRs react in a tire tread compound.
 Who are you hoping will benefit from your investigations? How significant are your results to the tire and automotive industry?
Who are you hoping will benefit from your investigations? How significant are your results to the tire and automotive industry?
When other researchers and compounders are aware of this different coupling behavior, they can better calculate the required amount of additional silane in a silica-filled compound with functionalized SBR. This will hopefully lead to a more optimized tire tread compound.
What takeaways can the audience expect from the presentation?
The takeaways encompass: an overview of the manifold reaction possibilities inside a modern tire tread compound, plus sharing our astonishment about the different reactivity of functionalized SBRs in a silica/silane-filled rubber compound.
Don’t miss Anke Blume’s presentation at Tire Technology Expo; timings to be announced soon!
 Biography
Biography
Anke studied chemistry at Leibniz University Hannover, Germany, and did her PhD thesis at the German Institute for Rubber Technology (DIK – Deutsches Institut für Kautschuktechnologie). She joined the Applied Technology department at Degussa (later Evonik Industries) in 1996, where she developed new silica and silanes for rubber applications, and in October 2013, she became the head of the Elastomer Technology and Engineering department at the University of Twente in the Netherlands.