Resins used as viscoelastic modifiers in tread rubber compounds could represent a paradigm shift in the development of passenger car tire tread formulations.
Resins have been traditionally used in tire compounding, for example, as tackifiers to improve building tack, as reinforcing resins to increase compound stiffness and hardness, and curing resins mostly for the manufacture of curing bladders. These applications and the corresponding types and chemistries are described extensively in numerous publications1-4. With the introduction of the EU regulation5 on safe oils and the banning of distilled aromatic extract (DAE) in 2010, there was a need to compensate for the reduction of wet traction of carbon black and silica-reinforced tread compounds when non-labeled process oils such as treated distilled aromatic extract (TDAE) were used instead6,7. The introduction of the regulation on tire labeling in Europe 20098 has put an additional focus on enhancing the wet traction performance of pneumatic tires.
There is a strong correlation between the glass transition temperature (Tg) and rubber characteristics such as wet traction9. Since the transition zone expressed by the Tg corresponds to the area of maximum hysteresis, it is an optimum area to achieve wet traction. The frequency of deformation of slipping rubber related to wet traction depends on the road surface roughness, and is reported in the range of 103 to 106Hz10. With the Williams-Landel-Ferry (WLF) transformation, this deformation frequency can be converted to temperature ranges. Common practice is the use of tan δ values at 0°C as a wet traction indicator from dynamic mechanical analysis (DMA) temperature sweep measurements11,12.
It is common practice in the rubber industry to blend rubbers with different macro and micro structures to realize performance profiles that are unachievable with one polymer alone. Since resins are oligomers with Tg ranging from +35 to +100°C, it was recognized early that they can be used to modify the visco-elastic responses of rubber-resin blends. Due to the higher Tg of the resins, the transition zone of these systems is shifted to higher temperatures and the dynamic elastic modulus of the rubbery plateau drops in DMA temperature sweep measurements13. The phenomenon was investigated in more detail with model resins14, 15.
As early as 1983, a patent application was filed disclosing the positive effect of resins on hysteresis16. However, due to the drop of the rubbery plateau (or G’) caused by the presence of resins and interpreted as a reduction of entanglement, the rolling resistance of these compounds was also affected. Therefore, the dosages of resins were kept at the lowest possible level to achieve the desired effect such as building tack. The announcement of the ban on aromatic oil DAE caused a paradigm change in the tire industry with an increasing number of patent filings claiming wet traction improvements through the use of resins in dosages which were far higher than known from typical resin applications17 (Figure 1). A number of resin classes are disclosed in various patent applications, for example petroleum-derived pure aromatic resins18, naturally-derived resins19, aromatically-modified terpene resins20, and also aromatically modified terpene phenol resins21. Terpene phenol resins seem to be of particular interest for the improvement of wet traction. In an application22 published in 2007, a silica-filled passenger car compound formulation is described, which comprises a terpene phenol resin with low OH number in combination with TDAE oil, a so-called ‘safe’ or non-labeled process oil, which passes the test method IP34623 referenced in the EU-regulation5 which became mandatory in 2010. Later, another patent application on the combination of terpene phenol resins and process oils, which comply with IP346, was filed in 200824. Both applications specify a range of OH numbers of the terpene phenol resin,indicating that a lower OH number is preferred22. Additional research and corresponding patent applications have been since filed25 related to the use of terpene phenol resins, with characteristics such as resin softening point and OH number as an additive to improve traction.
While the effort to get a better understanding of the interaction of resin with polymer on a molecular level continues26, the use of resins as a visco-elastic modifier has widely spread in the tire industry and represents an additional tool for the formulation of tread rubber compounds to meet the future requirements for road safety and fuel efficiency.
Kraton Corporation is a leading global producer of styrenic block copolymers, specialty polymers and high-value performance products derived from pine wood pulping co-products. Kraton’s polymers are used in a wide range of applications. Its products are sold to a wide range of customers in over 70 countries worldwide.
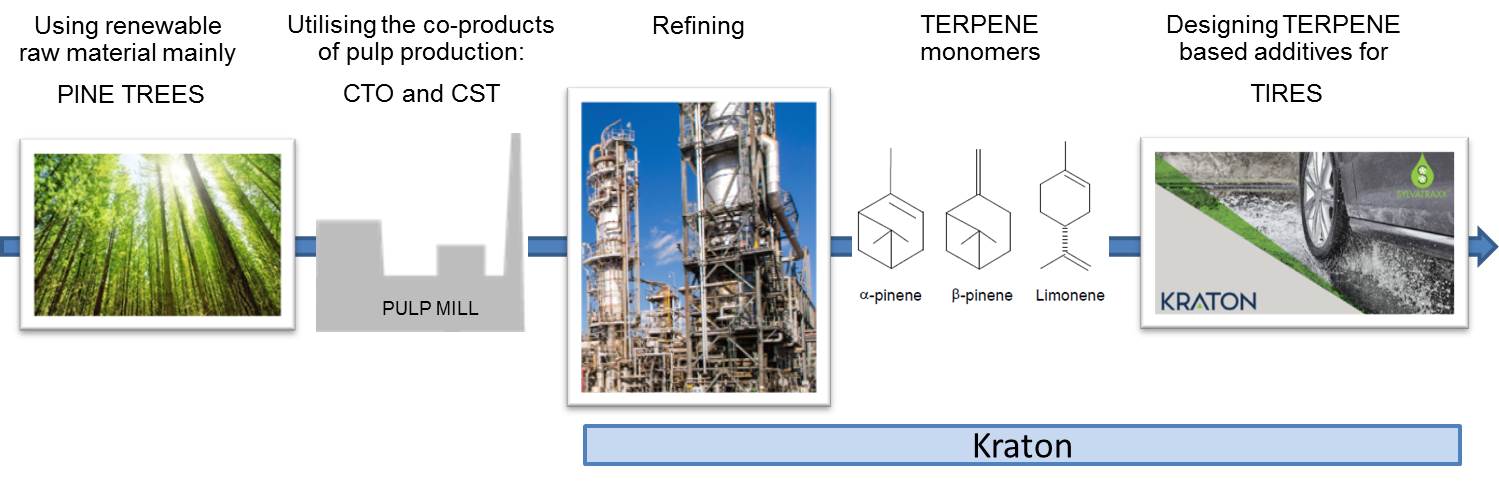 Value chain: tire performance additives from trees
Value chain: tire performance additives from trees
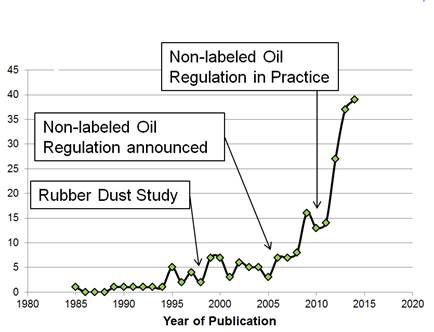
Number of patent applications related to the use of resin to enhance wet traction
References
1) Resins used in Rubber, P.O. Powers, Rubber Chemistry and Technology, 36, 1542-1570, 1963
2) Resins in Rubber; G.L.Brown; PICC Publ. (1969)
3) Entwicklung des Einsatzes van Harzen in der Gummiindustrie, H. Fries, GAK 38(9) 454, 1985
4) Rubber Compounding – Chemistry and Applications; Ed. Brendan Rogers; CRC Press (2016), p.
5) Commission Regulation (EC) No 552/2009 Annex XVII, entry 50.
6) John Wadie, New unlabelled Process Oils for the Tyres and Rubber Industry, Tyre Tech ’99 Prague
7) V. Null; Safe Process Oils for the Tires with Low Environmental Impact, KGK 52(12), 799, 1999
8) Regulation (EC) No 1229/2009 of the European Parliament and of the Council, Nov. 25, 2009
9) K.H.Nordsiek; Die Tg-Beziehung – Grundlagen und Bedeutung fuer die Kautschuktechnologie, KGK 39(7), p.599, 1986
10) C.M.Roland; Glass Transition in Rubbery Materials, Rubber Chem.Technol.85(3), 313, 2012
11) R. Sattelmeyer; Novolak Resins in Tread Compounds, KGK 47(9), p.659, 1994
12) N.Vleugels, W. Pille-Wolf, W.K.Dierkes, J.W.M.Noordermeeer; Understanding the Influence of Oligomeric resins on the Traction and Rolling Resistance of Silica-Reinforced Tire Treads, Rubber Chem. Technol. 88(1), 65-79 (2015)
13) D. W. Aubrey, M. Sherriff; Viscoelasticity of rubber-resin mixtures, J. Polym.Sci.Chem.Ed. 16(10), 2631, 1978
14) R.H.Schuster, G.Thielen, M.L.Hallersleben; Loeslichkeit und Verteilung von Kohlenwasserstof-Modellharzen in Kautschukverschnitten, KGK 44(3), 232, 1991
15) G. Thielen, R.H.Schuster, M.L.Hallensleben, Model Resins Leading to a Controlled Polymer Phase Morphology, KGK 46(4), 263, 1993
16) J.D.Wood, C.J.Stacy; US4373041, (Philipps Petroleum Company), 1983
17) W. Pille-Wolf; Tailored Sustainable Products for the Tire Industry; Tire Expo 2015, Cologne; slide 10
18) J.P. Lambotte, U.S.Patent 5877249 (Goodyear Tire & Rubber Company), 1999
19) G.Labauze, S.Mathieu; U.S.Patent 7084288 (Michelin Recherche & Technique S.A.), 2006
20) T.Kawamo; JP2562337 (Yokohama Rubber Corp.), 1996
21) W. Pille-Wolf, A.Deshpande US8637606 (Arizona Chemical Company LLC), 2014
22) W.Pille-Wolf, US2007037908 (Arizona Chemical Company LLC), 2007
23) IP346 test method, Energy Institute, http://publishing.energyinst.org/ip-test-methods/full-list-of-ip-test-methods-publications/ip-346-determination-of-polycyclic-aromatics-in-unused-lubricating-base-oils-and-asphaltene-free-petroleum-fractions-dimethyl-sulphoxide-extraction-refractive-index-method
24) S.Katou, EP2270088 (Bridgestone Corp.), 2013
25) E. Hotaling, X.Satigny, R. Stubblefield; WO2016109476 (Michelin Recherche & Technique S.A.), 2016
26) S. L’Heveder, F. Sportelli & N. A. Isitman (2016) Investigation of solubility in plasticised rubber systems for tire applications, Plastics, Rubber and Composites 45(7), 319-325, 2016



